Manufacturing and sales of medical devices
Medical devices approved (certified) by the MHLW (RCB) must be sold into the Japanese market by a MAH*1 who is responsible for quality control and post-market safety control.
If a foreign manufacturer does not have a MAH license, it must choose to distribute a medical device from the following options:
- Obtained a license of MAH
- Commission to a MAH
Foreign Special Approval System (FSAS) and Designated Marketing Authorization Holder (DMAH)
When a foreign manufacturer exporting a medical device to Japan appoints a MAH and applies to the MHLW (or RCB), the MHLW (or RCB) may grant special approval for it .
Through this system, foreign manufacturers have the following benefits:
- Approval (certification) can be held by itself.
- Focus on sales
It is possible to obtain a license of MAH at the Japan branch.
What is DMAH?
The marketing authorization holders is required to perform the following tasks.
- DMAH*2 conformities with ministerial ordinances on QMS*3 and GVP*4 on behalf of foreign manufacturers who have obtained a special foreign approval (certification).
- DMAH takes measures to prevent the occurrence of hazards in health and hygiene caused by the medical devices pertaining to such approval.
Schematic diagram of DMAH
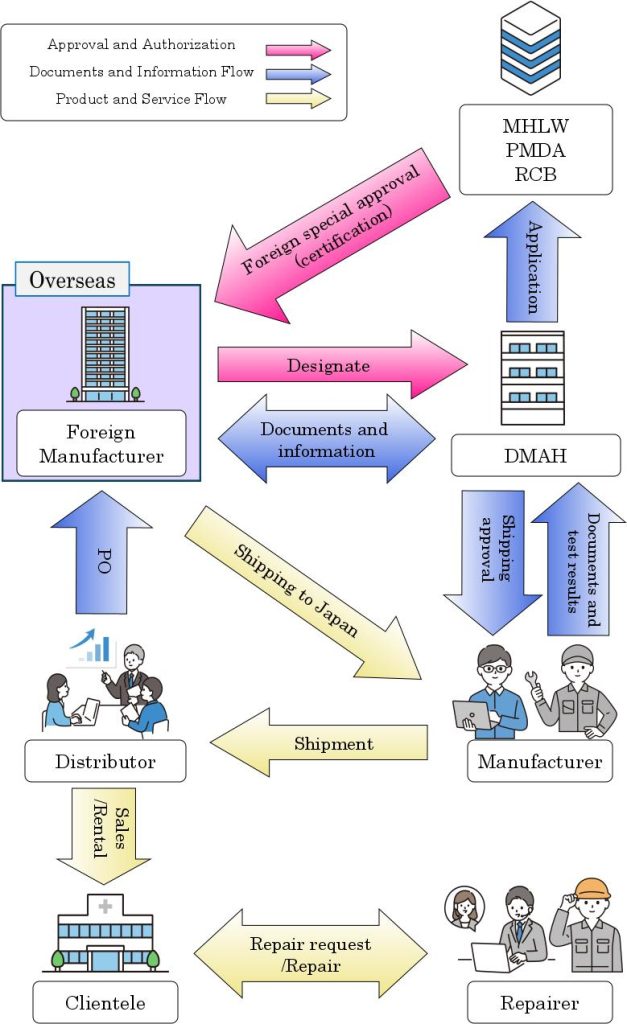
Outline of Designated Marketing Authorization Service
- Cooperation in manufacturing control and quality control.
- Post-marketing safety control.
- Representation and management of regulatory application, etc.
Please contact us for more information.
